Discover more about a career in Project Quality and Risk Management
Project Quality and Risk Management play a crucial role in ensuring the success of a clinical trial. Effective management of quality helps maintain data integrity, compliance with regulations, and reliable results. By identifying, assessing, and mitigating risks, potential issues can be addressed proactively, ensuring patient safety, adherence to timelines, and the overall success of the trial
What is Quality Management?
Quality Management in clinical trials refers to the systematic approach of ensuring that all clinical trial aspects follow established standards, regulations, and guidelines. It involves implementing processes and procedures to monitor, evaluate, and control various aspects of the trial, such as study protocols, data collection, participant safety, and documentation. Within Parexel, Project Quality and Risk Management builds Quality insights and risk mitigation into daily interaction. PQRM aims to ensure projects maintain the integrity and reliability of trial data while upholding participant rights and safety. It includes activities like quality control, quality assurance, risk assessment, mitigation, and effective communication and collaboration among stakeholders.
What is Risk Management?
Risk management in clinical trials involves identifying, assessing, and mitigating potential risks and uncertainties that could impact the successful conduct of the trial. It encompasses a systematic approach to proactively identify risks related to participant safety, data integrity, protocol adherence, regulatory compliance, and operational processes. Risk management involves conducting risk assessments to prioritize and evaluate identified risks, developing risk mitigation strategies, and implementing appropriate controls and preventive measures. The goal is to minimize the likelihood and impact of risks throughout the trial, ensuring participant safety, data quality, and overall trial success. Regular monitoring and review of risks are conducted, and adjustments are made to maintain effective risk management throughout the trial.
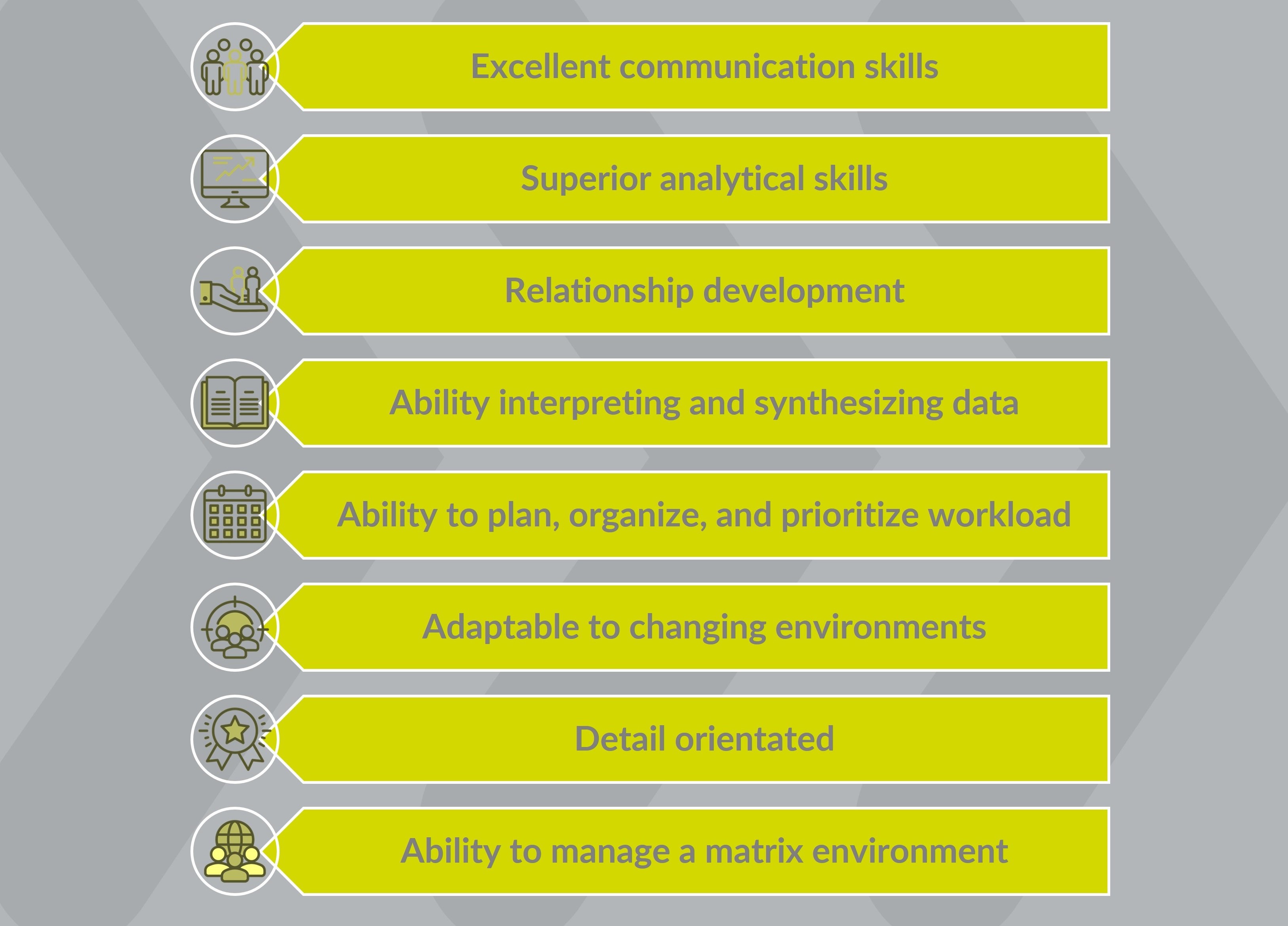
To be successful as a Project Quality and Risk Management leader in clinical research, several key skills are needed:
- Strong knowledge of quality and risk management principles and methodologies in clinical research.
- Excellent problem-solving and analytical skills to identify and assess potential risks and develop mitigation strategies.
- Thorough understanding of regulatory requirements and guidelines to ensure compliance throughout the trial.
- Effective communication and leadership skills to collaborate with cross-functional teams and stakeholders.
- Attention to detail and the ability to ensure data integrity and maintain high-quality standards.
- Adaptability and the ability to handle multiple projects simultaneously, while adhering to timelines and budgets.
- Strong decision-making skills to make informed choices when managing risks and quality issues.
- Continuous learning and keeping up-to-date with industry trends and advancements in risk management methodologies.
- Proficiency in project management tools and software to effectively manage and track project progress.
- Strong organizational and time management skills to prioritize tasks and ensure efficient project execution.
Meet Charity Poskonka
Charity has been with Parexel collectively for over 20 years, with more than 14 years within a Project Quality and Risk Mangement role.
Watch Charity's video to:
1) Find out a little more about what role she started in at Parexel.
2) Why she enjoys her role in PQRM.
3) Discover the opportunities for growth.
Featured Jobs
- Director, Quality Assurance - Singapore - Remote
- Senior Issue Lead - FSP Argentina - Remote; Brazil, Remote; Mexico, Remote, Remote
- Asset Quality Lead, Director - FSP Argentina - Remote; Brazil, Remote; Mexico, Remote, Remote
Find Out More
Sign up for our Talent Community
Sign up and we’ll reach out with job alerts when positions that match your career interests become available. We’ll also share periodic updates about the latest company news and events.




