Ensuring Regulatory Excellence
As the largest and most experienced regulatory consultancy group globally, Parexel offers unparalleled expertise and a vast global reach. Our comprehensive services span from product profiling and regulatory strategy to compliance, publishing and submission, commercialization planning, and ongoing lifecycle management support. Our teams are dedicated to maximizing success in drug development, ensuring new treatments reach patients faster.
Our progressive integration of clinical and regulatory consulting expertise enables our teams to engage in a variety of innovative studies, continually expanding their knowledge and driving forward the frontiers of medical science. Join us at Parexel, where collaboration, passion, and excellence converge to transform patient care.
"Parexel has enabled me to gain experience across a variety of diseases and drug products while strengthening and broadening my knowledge of regulatory affairs. The variety of my work, along with support from my colleagues and manager, allows me to continue learning and supports my professional growth."
- Liam Fitzgerald, Senior Regulatory Affairs Associate
Our Approach to Regulatory Effectiveness
We achieve regulatory excellence through three focused teams: Regulatory Affairs & Operations, Compliance and Regulatory Strategy. Learn about our teams and how they work every day With Heart™.
Regulatory Affairs & Opperations
At Parexel, our Regulatory Affairs and Operations team ensures that regulatory submissions and activities adhere to the guidelines and regulations set by global health authorities, including the US Food and Drug Administration (US-FDA), European Medicines Agency (EMA), and National Medicinal Products Administration (NMPA). By developing unique regulatory strategies, securing submission approvals, and maintaining compliance for trials and products, our team plays a pivotal role in regulatory success. Joining our regulatory team means you'll gain experience across a variety of projects and therapeutic areas, including Orphan Drug, Rare Disease, OTC Drug, Cell and Gene Therapies (C>), and more.
Jessica Jung
Associate Project Director
"At Parexel, prioritizing patients is not just a directive; it is a way of life for each employee. This shared dedication is evident in our daily practices, extending beyond words to influence our actions and decisions. We understand that the work we do has a profound impact on people's lives, and this understanding is reflected in our relentless pursuit of excellence."
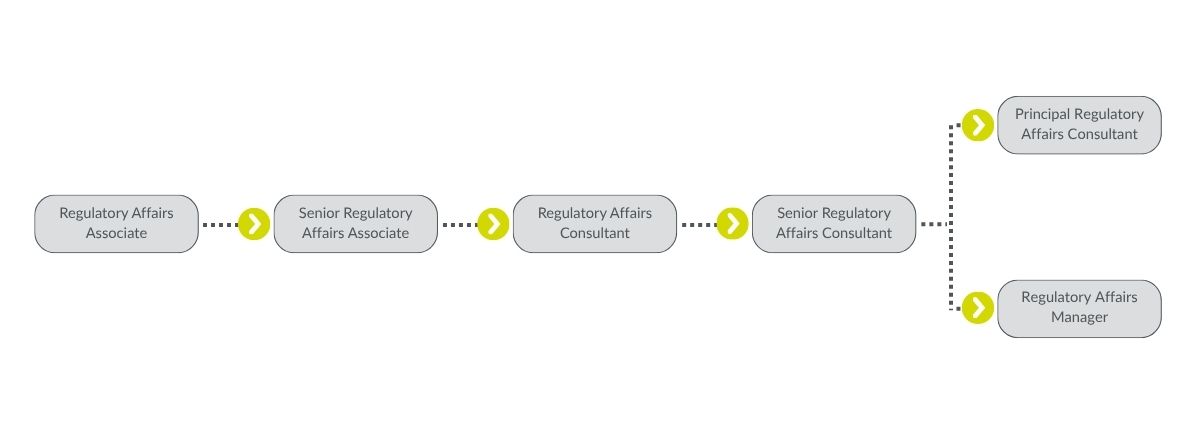
Regulatory Affairs Career Paths
From entry-level positions to senior leadership roles, the Regulatory Affairs team offers numerous opportunities for growth and specialization. Discover the different career paths and how you can make a lasting impact while advancing your career in this critical and ever-evolving field.
Compliance
Parexel’s Compliance Team combines a wealth of regulatory compliance knowledge and expertise to support companies globally with building cultures of compliance, while mitigating risks, managing remediations, and restoring client reputations. Our goal is to tailor solutions that make sense for our clients and the patients they serve. By fostering collaboration and leveraging diverse skills, we provide clients with strategic compliance guidance, inspection readiness, inspections support, a wide range of GxP auditing services, data integrity, training, and building quality management systems. We help clients navigate complex regulatory landscapes while maintaining high standards of quality and compliance.
Kevin Nolan
Principal Consultant
"The unique blend found in the Parexel Strategic Compliance Consulting team of extensive regulatory knowledge and industry experience by former FDA investigators and industry experts truly sets Parexel apart. Our team brings a deep understanding of the Regulatory landscape, coupled with a practical perspective on the challenges faced by companies in the life sciences industry. Parexel’s ability to navigate complex regulatory requirements while considering the operational and business aspects of clients enables Parexel to offer strategic and efficient solutions."

Through leveraging our unique fusion of scientific, regulatory, and business proficiency the Compliance group applies unparalleled breadth and depth of expertise that offer services to create and maximize product value for clients throughout the product lifecycle. Uncover how Jo Wang has leveraged her FDA expertise to rise to VP Technical at Parexel, where she exemplifies our "With Heart" value daily, helping to accelerate medicine delivery.
Regulatory Strategy
Development Programs and Insights
We design targeted learning to boost your success at work. Our tailored programs and resources specifically address our industry and the unique needs of your development.
Review our most relevant and popular development areas:
Explore the fascinating world of clinical research as our Regulatory experts uncover the latest trends, industry updates, and valuable insights of the ever-evolving regulatory landscape.
- Parexel Announces AI Partnership with Weave Bio to Accelerate Regulatory Submission Processes | Parexel International Corporation
- New FDA regulatory initiatives improving rare disease drug development
- Fast-track to UK market: Mastering the MHRA's recognition procedure
- Parexel announces appointments of two FDA luminaries – Lola Fashoyin-Aje, M.D., MPH and Tala Fakhouri, Ph.D., MPH – to its Consulting team, further strengthening regulatory, medical and AI expertise
- RNA-based therapies: Aligning CMC strategies with “borrowed” FDA regulatory guidance
- Optimize your CMC strategy: Five steps to avoid regulatory setbacks
Featured Jobs
Looking to make an impact in regulatory affairs? Discover our diverse range of regulatory positions. From entry-level to executive roles, find where you fit in our innovative team. Each opportunity offers the chance to influence global healthcare and grow your career. Explore our top regulatory openings and take your next career step with Parexel!
Featured Jobs
- Regulatory Affairs Consultant - Labelling Germany - Remote; Switzerland, Remote, Remote
- Senior Regulatory Affairs Consultant- CMC Biologics - India - Remote
- Regulatory Affairs Consultant - E2E Labelling - Berlin, Germany
- Senior Regulatory Affairs Associate - India - Remote
- Senior Regulatory Affairs Associate - Labeling Compliance Analytics - United States of America - Remote
- Senior Regulatory Affairs Associate - Labeling Compliance - United States of America - Remote
Sign up for our Talent Community
Sign up and we’ll reach out with job alerts when positions that match your career interests become available. We’ll also share periodic updates about the latest company news and events.











