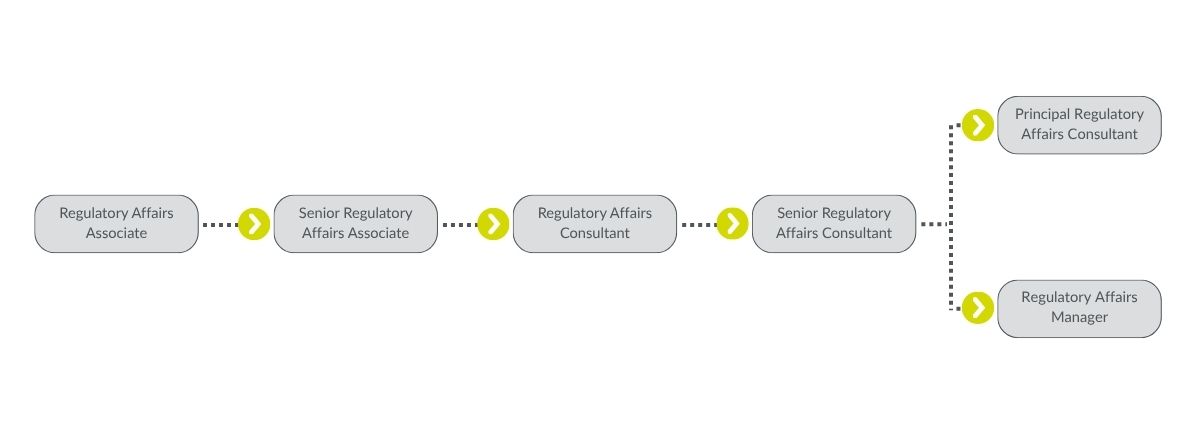
Ensuring Regulatory Excellence
As the largest and most experienced regulatory consultancy group globally, Parexel offers unparalleled expertise and a vast global reach. Our comprehensive services span from product profiling and regulatory strategy to compliance, publishing and submission, commercialization planning, and ongoing lifecycle management support. Our teams are dedicated to maximizing success in drug development, ensuring new treatments reach patients faster.
Our progressive integration of clinical and regulatory consulting expertise enables our teams to engage in a variety of innovative studies, continually expanding their knowledge and driving forward the frontiers of medical science. Join us at Parexel, where collaboration, passion, and excellence converge to transform patient care.
"Parexel has enabled me to gain experience across a variety of diseases and drug products while strengthening and broadening my knowledge of regulatory affairs. The variety of my work, along with support from my colleagues and manager, allows me to continue learning and supports my professional growth."
- Liam Fitzgerald, Senior Regulatory Affairs Associate
Our Approach to Regulatory Effectiveness
We achieve regulatory excellence through three focused teams: Regulatory Affairs & Operations, Compliance and Regulatory Strategy. Learn about our teams and how they work every day With Heart™.
Regulatory Affairs & Opperations
At Parexel, our Regulatory Affairs and Operations team ensures that regulatory submissions and activities adhere to the guidelines and regulations set by global health authorities, including the US Food and Drug Administration (US-FDA), European Medicines Agency (EMA), and National Medicinal Products Administration (NMPA). By developing unique regulatory strategies, securing submission approvals, and maintaining compliance for trials and products, our team plays a pivotal role in regulatory success. Joining our regulatory team means you'll gain experience across a variety of projects and therapeutic areas, including Orphan Drug, Rare Disease, OTC Drug, Cell and Gene Therapies (C>), and more.
Intro/Headline Here
Hilitia num non pro cus sed. Tut magnita turectati nossinus, seribus volorest ium aut quam. Unis ut aut la corerio modis. Hilitia num non pro cus sed.
If applicable, a bulleted list could go here:
- Typi non habent claritatem insitam; est usus legentis
- Bin iis qui facit eorum claritatem.
- Claritas est etiam processus dynamicus.
- Quam nunc putamus parum claram, anteposuerit litterarum
Title Goes Here
This is where a subhead woud go - qui facit eorum claritatem.
Typi non habent claritatem insitam; sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore magna aliquam erat volutpat. Ut wisi enim ad minim veniam, quis nostrud exerci tation ullamcorper suscipit lobortis nisl ut aliquip ex ea commodo consequat.
If applicable, a bulleted list could go here:
- Typi non habent claritatem insitam; est usus legentis
- Bin iis qui facit eorum claritatem. Investigationes demonstraverunt. Eodem modo typi, qui nunc nobis videntur.
- Electores legere me lius quod ii legunt saepius
- Claritas est etiam processus dynamicus, qui sequitur mutationem consuetudium lectorum. Mirum est notare quam littera gothica.
- Quam nunc putamus parum claram, anteposuerit litterarum
- Quam nunc putamus parum claram, anteposuerit litterarum
Compliance
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Integer hendrerit et nunc non auctor. Vestibulum quis commodo arcu, dapibus varius mauris. Sed in turpis dolor. Maecenas blandit, neque nec tincidunt rhoncus, risus mi pretium sapien, tincidunt elementum leo lacus sit amet elit. Nunc et felis quis erat accumsan condimentum.
Kevin Nolan
Principal Consultant
"The unique blend found in the Parexel Strategic Compliance Consulting team of extensive regulatory knowledge and industry experience by former FDA investigators and industry experts truly sets Parexel apart. Our team brings a deep understanding of the Regulatory landscape, coupled with a practical perspective on the challenges faced by companies in the life sciences industry. Parexel’s ability to navigate complex regulatory requirements while considering the operational and business aspects of clients enables Parexel to offer strategic and efficient solutions."

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Integer hendrerit et nunc non auctor. Vestibulum quis commodo arcu, dapibus varius mauris. Sed in turpis dolor. Maecenas blandit, neque nec tincidunt rhoncus, risus mi pretium sapien, tincidunt elementum leo lacus sit amet elit. Nunc et felis quis erat accumsan condimentum.
Regulatory Strategy
TRABAJO QUE PRIORIZA A LOS PACIENTES
Diseñamos un aprendizaje dirigido para aumentar su éxito en el trabajo. Nuestros programas y recursos personalizados abordan específicamente nuestra industria y las necesidades únicas de su desarrollo.
Revise nuestras áreas de desarrollo más relevantes y populares:
This is where a subhead woud go - qui facit eorum claritatem.
Typi non habent claritatem insitam; sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore magna aliquam erat volutpat. Ut wisi enim ad minim veniam, quis nostrud exerci tation ullamcorper suscipit lobortis nisl ut aliquip ex ea commodo consequat.
If applicable, a bulleted list could go here:
- Typi non habent claritatem insitam; est usus legentis
- Bin iis qui facit eorum claritatem. Investigationes demonstraverunt. Eodem modo typi, qui nunc nobis videntur.
- Electores legere me lius quod ii legunt saepius
- Claritas est etiam processus dynamicus, qui sequitur mutationem consuetudium lectorum. Mirum est notare quam littera gothica.
- Quam nunc putamus parum claram, anteposuerit litterarum
- Quam nunc putamus parum claram, anteposuerit litterarum
Professional and Personal development Spotlight
This is where a subhead woud go - qui facit eorum claritatem.
Typi non habent claritatem insitam; sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore magna aliquam erat volutpat. Ut wisi enim ad minim veniam, quis nostrud exerci tation ullamcorper suscipit lobortis nisl ut aliquip ex ea commodo consequat.
If applicable, a bulleted list could go here:
- Typi non habent claritatem insitam; est usus legentis
- Bin iis qui facit eorum claritatem. Investigationes demonstraverunt. Eodem modo typi, qui nunc nobis videntur.
- Electores legere me lius quod ii legunt saepius
- Claritas est etiam processus dynamicus, qui sequitur mutationem consuetudium lectorum. Mirum est notare quam littera gothica.
- Quam nunc putamus parum claram, anteposuerit litterarum
- Quam nunc putamus parum claram, anteposuerit litterarum
Featured Jobs
- Regulatory Affairs Consultant: Medical Device and Combination Product Expert - Milán, Italia
- CMC薬事シニアアソシエイト(FSPモデル) - Japan - Remote
- 開発薬事(シニア)コンサルタント(FSPモデル) - Japan - Remote
- Regulatory Affairs Associate - Bangalore, India
- Senior Project Specialist (PC) - Petaling Jaya, Malasia
- Senior Regulatory Affairs Associate - Bangalore, India
Suscribirse a nuestra Comunidad de Talento
Suscríbase y le enviaremos alertas de trabajos cuando haya puestos de trabajo disponibles que coincidan con sus intereses profesionales. Además, compartiremos actualizaciones periódicas sobre las noticias y los eventos más recientes de la empresa.







